Pediatric emergency medicine trisk 542
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (158.76 KB, 4 trang )
The peak age for urethral prolapse in prepubertal children is 5 to 8
years.
The majority of children with urethral prolapse present with vaginal
bleeding.
A doughnut-shaped protrusion from the vulva is found in urethral
prolapse.
Prompt attention is needed to correct the prolapse to avoid tissue
necrosis.
Urethral prolapse is the protrusion of the distal urethral mucosa outward
through its meatus, with a cleavage plane between the longitudinal and circularoblique smooth-muscle layers of the urethra. Most prolapses happen
spontaneously, but some episodes are noted to have occurred following a sudden
or recurrent increase in intra-abdominal pressure (coughing, straining with
constipation, lifting heavy objects). The prolapsed segment is constricted at the
meatus and venous blood flow is impaired, so the involved tissue becomes
swollen, edematous, and dark red or purplish. If the urethral prolapse is not
corrected, the tissue can become thrombosed and necrotic.
About half of affected females are prepubertal children, while the majority of
the remainder are postmenopausal women. Most urethral prolapses during
childhood occur between the ages of 2 and 10 years, with the peak at 5 to 8 years
of age. The majority of prepubertal children with urethral prolapse are African
Americans.
Clinical Manifestations
Vaginal bleeding or spotting is the chief complaint of 90% of children with
significant urethral prolapses. The bleeding is painless, occasionally
misinterpreted as hematuria or menstruation, and is sometimes accompanied by
urinary frequency or dysuria. On examination of the child’s perineum, a red or
purplish, soft, doughnut-shaped mass is seen ( Fig. 92.6 ). Most prolapses are not
tender and measure 1 to 2 cm in diameter. By retracting the labia majora
posterolaterally, the examiner can often demonstrate that the mass is separate
from and anterior to the vaginal introitus; this process may be difficult if the
prolapse is large. A small central dimple in the mass indicates the urethral lumen,
though the dimple can be missed if lighting is inadequate, bleeding is active, or
mucosal edema is significant. In most cases, the appearance of the prolapse is
diagnostic. However, if the diagnosis is in doubt, sterile straight catheterization of
the bladder through the mass can be performed to demonstrate the anatomic
relationships safely and rapidly. Urinalysis may show red blood cells and urine
cultures are routinely sterile, though these tests may not be clinically indicated if
the child otherwise looks well. Urethral polyps, prolapsed ureterocele, sarcoma
botryoides, and urethral carcinoma may be included in the differential diagnosis,
but they are rare in children and lack the characteristically annular appearance of
a urethral prolapse.
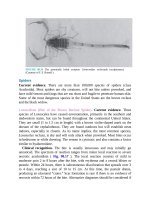
FIGURE 92.6 A : Urethral prolapse in a 6-year-old girl with “vaginal” bleeding. The vaginal
orifice cannot be seen. B : The smooth doughnut shape and central lumen are characteristic
features of a urethral prolapse, which if large or swollen, often conceals the vagina below it.
Management
For the symptomatic patient with a small segment of prolapsed mucosa that is not
necrotic, warm moist compresses or sitz baths, combined with a 2-week course of
topical estrogen cream, may be prescribed. Most patients treated in this way have
improved within 10 to 14 days and remained normal thereafter, thus avoiding
surgery. Patients with dark-red or necrotic mucosa should be treated surgically
within several days by reduction of the prolapse and/or excision of necrotic
tissue. After the diagnosis is confirmed by cystoscopy, the prolapse is excised and
the cut edges are sutured together. It is also important for the practitioner to
address any precipitant related to the prolapse such as chronic constipation or
other Valsalva-related intra-abdominal strain.
Manual separation of adhesions should be avoided.
Contraceptive Devices
Background
Use of long-acting reversible contraceptive (LARC) devices among adolescent
patients has increased significantly over the past decade. In 2002, only 2.4% of
young women used these devices, but by 2013 more than 11.6% were using one
of these methods. There are two main types of LARC devices—IUDs and
subdermal hormonal implants. Although side effects are rare, ED clinicians
should be aware of complications that represent potential clinical emergencies.
CLINICAL PEARLS AND PITFALLS
Recognize when patients who have a contraceptive device in place
may be presenting with symptoms suggestive of a complication.
Provide guidance on the optimal process for evaluating complications
related to use of a contraceptive device.
INTRAUTERINE DEVICES
Clinical Manifestations
Serious complications related to having a contraceptive device place are rare.
Among IUD users, rare, but serious side effects include pregnancy, uterine
perforation, expulsion, and infection. Pregnancies with an in situ IUD have a
higher risk of being an ectopic pregnancy and, if the IUD is left in place, women
are more likely to experience a spontaneous abortion or prolonged bleeding.
The risk of perforation is low, and is estimated to be 1 in 1,000; most occur
within 2 months of insertion. Clinical symptoms of perforation include persistent
or worsening pain, bleeding, hematuria, abdominal distention, and fever.
Perforations are usually fundal and, given that the device has no sharp edges and
that no incisions or sharp instruments are placed in the uterus during the
procedure, are generally not associated with hemorrhage or damage to internal
visceral organs. However, cervical perforation or lateral perforation at the level of
the internal cervical os or within the uterus can result in vascular disruption with
associated hemodynamic changes, including hemodynamic instability. Anterior
perforation may result in damage to the bladder, which may present with
suprapubic pain, dysuria, or persistent vaginal leakage of fluid. The diagnosis
may be made based on clinical symptoms or with an ultrasound. Abdominal as
well as transvaginal images are generally necessary to confirm the diagnosis, with
3D ultrasound providing greater sensitivity, particularly in obese women and
those with subtle problems of positioning, like embedment of the long arms of the
device within the myometrium.
Expulsion occurs in approximately 2% of IUD placements. The risk is highest
among nulliparous women. The symptoms of expulsion include persistent
abdominal discomfort following insertion that is not improving over time, or
worsening pain that suddenly resolved. Rarely, women may identify the device
following expulsion; more commonly, the device is only partially expulsed
resulting in ongoing pain.
Infection occurs following 1% of IUD placements and is most likely during 21
days following IUD placement. Patients with symptoms of vaginitis should be
evaluated for an STD, bacterial vaginosis, or a vulvovaginal yeast infection and
treated accordingly.
Management
Management of pregnancies with an in situ IUD depends on the woman’s desire
to continue or terminate the pregnancy, gestational age, IUD location, and
whether IUD strings are visible. The U.S. Food and Drug Administration, the
Centers for Disease Control, and the American College of Obstetricians and
Gynecologists (ACOG) recommend that the IUD be removed from a pregnant
woman as soon as possible, if the strings are visualized or if the IUD is in the
cervix.
If perforation is confirmed by ultrasound, and the patient recently had the
device placed (<24 hours) should be observed for hemodynamic compromise,
which would require surgical intervention. In the absence of vascular injury, the
patient can be referred to adolescent medicine or gynecology for device removal.
Rarely, an IUD is noted to be outside the uterus on imaging. If there are concerns
for visceral injury, the patient should be evaluated by gynecology or pediatric
surgery. If there are no concerns for visceral injury, the patient can be referred to
gynecology of general surgery for device removal, which can generally be
performed laparoscopically. If an ultrasound is performed and the device cannot
be located within the uterus, an x-ray or CT of the chest, abdomen, and pelvis can
be performed to locate the device, which is radiopaque. ACOG has an algorithm
for managing IUDs with lost strings and therefore an IUD of unknown location.
An x-ray is inexpensive and can be obtained fairly rapidly.
A partially expulsed IUD may be located within the vagina or the cervical os.
Removal is straightforward and can be performed by an ED provider using ring
forceps, a Kelly clamp, or another grasping device. After removal, the IUD
should be inspected to confirm that the entire device was removed.