Tài liệu Causal Agents docx
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (537.47 KB, 19 trang )
Causal Agents:
Schistosomiasis is caused by digenetic blood trematodes. The three main species
infecting humans are Schistosoma haematobium, S. japonicum, and S. mansoni.
Two other species, more localized geographically, are S. mekongi and S.
intercalatum. In addition, other species of schistosomes, which parasitize birds and
mammals, can cause cercarial dermatitis in humans.
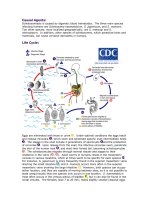
Life Cycle:
Eggs are eliminated with feces or urine . Under optimal conditions the eggs hatch
and release miracidia , which swim and penetrate specific snail intermediate hosts
. The stages in the snail include 2 generations of sporocysts and the production
of cercariae . Upon release from the snail, the infective cercariae swim, penetrate
the skin of the human host , and shed their forked tail, becoming schistosomulae
. The schistosomulae migrate through several tissues and stages to their
residence in the veins ( , ). Adult worms in humans reside in the mesenteric
venules in various locations, which at times seem to be specific for each species .
For instance, S. japonicum is more frequently found in the superior mesenteric veins
draining the small intestine , and S. mansoni occurs more often in the superior
mesenteric veins draining the large intestine . However, both species can occupy
either location, and they are capable of moving between sites, so it is not possible to
state unequivocally that one species only occurs in one location. S. haematobium
most often occurs in the venous plexus of bladder , but it can also be found in the
rectal venules. The females (size 7 to 20 mm; males slightly smaller) deposit eggs
in the small venules of the portal and perivesical systems. The eggs are moved
progressively toward the lumen of the intestine (S. mansoni and S. japonicum) and
of the bladder and ureters (S. haematobium), and are eliminated with feces or urine,
respectively . Pathology of S. mansoni and S. japonicum schistosomiasis includes:
Katayama fever, hepatic perisinusoidal egg granulomas, Symmers’ pipe stem
periportal fibrosis, portal hypertension, and occasional embolic egg granulomas in
brain or spinal cord. Pathology of S. haematobium schistosomiasis includes:
hematuria, scarring, calcification, squamous cell carcinoma, and occasional embolic
egg granulomas in brain or spinal cord.
Human contact with water is thus necessary for infection by schistosomes. Various
animals, such as dogs, cats, rodents, pigs, hourse and goats, serve as reservoirs for
S. japonicum, and dogs for S. mekongi.
Geographic Distribution:
Schistosoma mansoni is found in parts of South America and the Caribbean, Africa,
and the Middle East; S. haematobium in Africa and the Middle East; and S.
japonicum in the Far East. Schistosoma mekongi and S. intercalatum are found
focally in Southeast Asia and central West Africa, respectively.
Causal Agents:
Schistosomiasis is caused by digenetic blood trematodes. The three main species
infecting humans are Schistosoma haematobium, S. japonicum, and S. mansoni.
Two other species, more localized geographically, are S. mekongi and S.
intercalatum. In addition, other species of schistosomes, which parasitize birds and
mammals, can cause cercarial dermatitis in humans.
Life Cycle:
Eggs are eliminated with feces or urine . Under optimal conditions the eggs hatch
and release miracidia , which swim and penetrate specific snail intermediate hosts
. The stages in the snail include 2 generations of sporocysts and the production
of cercariae . Upon release from the snail, the infective cercariae swim, penetrate
the skin of the human host , and shed their forked tail, becoming schistosomulae
. The schistosomulae migrate through several tissues and stages to their
residence in the veins ( , ). Adult worms in humans reside in the mesenteric
venules in various locations, which at times seem to be specific for each species .
For instance, S. japonicum is more frequently found in the superior mesenteric veins
draining the small intestine , and S. mansoni occurs more often in the superior
mesenteric veins draining the large intestine . However, both species can occupy
either location, and they are capable of moving between sites, so it is not possible to
state unequivocally that one species only occurs in one location. S. haematobium
most often occurs in the venous plexus of bladder , but it can also be found in the
rectal venules. The females (size 7 to 20 mm; males slightly smaller) deposit eggs
in the small venules of the portal and perivesical systems. The eggs are moved
progressively toward the lumen of the intestine (S. mansoni and S. japonicum) and
of the bladder and ureters (S. haematobium), and are eliminated with feces or urine,
respectively . Pathology of S. mansoni and S. japonicum schistosomiasis includes:
Katayama fever, hepatic perisinusoidal egg granulomas, Symmers’ pipe stem
periportal fibrosis, portal hypertension, and occasional embolic egg granulomas in
brain or spinal cord. Pathology of S. haematobium schistosomiasis includes:
hematuria, scarring, calcification, squamous cell carcinoma, and occasional embolic
egg granulomas in brain or spinal cord.
Human contact with water is thus necessary for infection by schistosomes. Various
animals, such as dogs, cats, rodents, pigs, hourse and goats, serve as reservoirs for
S. japonicum, and dogs for S. mekongi.
Geographic Distribution:
Schistosoma mansoni is found in parts of South America and the Caribbean, Africa,
and the Middle East; S. haematobium in Africa and the Middle East; and S.
japonicum in the Far East. Schistosoma mekongi and S. intercalatum are found
focally in Southeast Asia and central West Africa, respectively.
Causal Agent:
The nematode (roundworm) Gnathostoma spinigerum and Gnathostoma hispidum,
which infects vertebrate animals. Human gnathostomiasis is due to migrating
immature worms.
Life Cycle:
Adapted from a drawing provided by Dr. Sylvia Paz Díaz Camacho, Universidade Autónoma de Sinaloa, Mexico.
In the natural definitive host (pigs, cats, dogs, wild animals) the adult worms reside
in a tumor which they induce in the gastric wall. They deposit eggs that are
unembryonated when passed in the feces . Eggs become embryonated in water,
and eggs release first-stage larvae . If ingested by a small crustacean (Cyclops,
first intermediate host), the first-stage larvae develop into second-stage larvae .
Following ingestion of the Cyclops by a fish, frog, or snake (second intermediate
host), the second-stage larvae migrate into the flesh and develop into third-stage
larvae . When the second intermediate host is ingested by a definitive host, the
third-stage larvae develop into adult parasites in the stomach wall . Alternatively,
the second intermediate host may be ingested by the paratenic host (animals such
as birds, snakes, and frogs) in which the third-stage larvae do not develop further
but remain infective to the next predator . Humans become infected by eating
undercooked fish or poultry containing third-stage larvae, or reportedly by drinking
water containing infective second-stage larvae in Cyclops .
Geographic Distribution:
Asia, especially Thailand and Japan; recently emerged as an important human
parasite in Mexico
Causal Agents:
The trematodes Fasciola hepatica (the sheep liver fluke) and Fasciola gigantica,
parasites of herbivores that can infect humans accidentally.
Life Cycle:
Immature eggs are discharged in the biliary ducts and in the stool . Eggs become
embryonated in water , eggs release miracidia , which invade a suitable snail
intermediate host , including the genera Galba, Fossaria and Pseudosuccinea. In
the snail the parasites undergo several developmental stages (sporocysts , rediae
, and cercariae ). The cercariae are released from the snail and encyst as
metacercariae on aquatic vegetation or other surfaces. Mammals acquire the
infection by eating vegetation containing metacercariae. Humans can become
infected by ingesting metacercariae-containing freshwater plants, especially
watercress . After ingestion, the metacercariae excyst in the duodenum and
migrate through the intestinal wall, the peritoneal cavity, and the liver parenchyma
into the biliary ducts, where they develop into adults . In humans, maturation
from metacercariae into adult flukes takes approximately 3 to 4 months. The adult
flukes (Fasciola hepatica: up to 30 mm by 13 mm; F. gigantica: up to 75 mm) reside
in the large biliary ducts of the mammalian host. Fasciola hepatica infect various
animal species, mostly herbivores.
Geographic Distribution:
Fascioliasis occurs worldwide. Human infections with F. hepatica are found in areas
where sheep and cattle are raised, and where humans consume raw watercress,
including Europe, the Middle East, and Asia. Infections with F. gigantica have been
reported, more rarely, in Asia, Africa, and Hawaii.
Causal Agents:
The nematode (roundworm) Angiostrongylus cantonensis, the rat lungworm, is the
most common cause of human eosinophilic meningitis. In addition, Angiostrongylus
(Parastrongylus) costaricensis is the causal agent of abdominal, or intestinal,
angiostrongyliasis.
Life Cycle:
Adult worms of A. cantonensis live in the pulmonary arteries of rats. The females lay
eggs that hatch, yielding first-stage larvae, in the terminal branches of the
pulmonary arteries. The first-stage larvae migrate to the pharynx, are swallowed,
and passed in the feces. They penetrate, or are ingested by, an intermediate host
(snail or slug). After two molts, third-stage larvae are produced, which are infective
to mammalian hosts. When the mollusk is ingested by the definitive host, the third-
stage larvae migrate to the brain where they develop into young adults. The young
adults return to the venous system and then the pulmonary arteries where they
become sexually mature. Of note, various animals act as paratenic (transport)
hosts: after ingesting the infected snails, they carry the third-stage larvae which can
resume their development when the paratenic host is ingested by a definitive host.
Humans can acquire the infection by eating raw or undercooked snails or slugs
infected with the parasite; they may also acquire the infection by eating raw produce
that contains a small snail or slug, or part of one. There is some question whether or
not larvae can exit the infected mollusks in slime (which may be infective to humans
if ingested, for example, on produce). The disease can also be acquired by ingestion