Pharmaceutical Substances Syntheses, Patents, Applications - Part 93 ppt
Bạn đang xem bản rút gọn của tài liệu. Xem và tải ngay bản đầy đủ của tài liệu tại đây (245.88 KB, 10 trang )
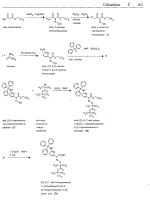
Fluvastatin sodium
F
921
nortropine
N-(8-fluoroethyl)-
nortropine
N-(p-fluorocthyl)nar-
tropine benzilate (V)
V+N b
Flutropium bromide
Reference(s):
DE
2
540 633 (Boehringer Ing.; appl. 12.9.1976).
Banholzer,
R.
et al.: Arzneim Forsch. (ARZNAD)
36,
1161 (1986).
synthesis
of
nortropine benzilate:
Bertholdt,
H.
et al.: Arzneim Forsch. (ARZNAD)
17,
719 (1967)
Trade Name(s):
I:
Flubron (S. S. Pharm.)
Fluvastatin sodium
(SRI-62320; XU-62-320; XU-620)
ATC: B04AB04
Use: hyperlipidemic, HMG-CoA-
reduclase inhibitor
RN: 93957-55-2 MF: C,H2,FNNa04 MW: 433.46
CN:
[R*.S*-(E)]-(~)-7-[3-(4-fluorophenyl)-I-(l-methylethyl)-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoic
acid
monosodium salt
free
acid
RN: 93957-54-
1
MF:
C2,HZ6FNO4
MW:
41 1.47
1.
HAI[CH2CH(CH3)2]2
toluene. THF,
-78
'C
2.
MnD2,
Et20
b
1.
diisobutylalurninum
hydride
ethyl 3-(4-fluoro-
phenyl)-1 -isopropyl-
indale-2-corboxylate
3-(4-fluor0phenyl)-
1
-isopropylindole-
2-carboxoldehyde
(I)
922
F
Fluvastatin sodium
2-(tributylstannyl)vinyl
ethyl ether
H3Cy~~3
THF, -78 OC
___,
(E)-3-[3-(4-fluoro-
phenyl)-1 -isopropyl-
indol-2-yl]-
propenal (11)
methyl
ocetoacetate
methyl (f)-(E)-7-[3-(4-fluoro-
phenyl)-1 -isopropylindol-
2-yl]-5-hydroxy-3-oxohept-
6-enoate
(ID)
I.
(H,c),c-NH,
.
BH,.
C2H50H H3Cy~~3
2.
separation of the diastereorners
I11
/
\
-
w0
F
0-CH3
methyl
(+)-erythro-(~)-3.5-dih~drax~-
7-[3-(4-fluorophenyl)-
1
-isopropyl-
indol-Z-yllhept-6-enoate
(IV)
*O
-
ONa
F
Fluvastatin
sodium
Fluvastatin
sodium
F
92
NoH.
BuLi.
THF
"
+
-
aOH,
H3C
0
CH-
I
tert-butyl
acetometote
tert-butyl (2)-(E)-7-[3-(4-lluoro-
phenyl).
1
-isopropylind0l-2-~l]-
5-hydroxy-3-oxohept-6-enoate
(VI)
1.
CH,OB(C,H,),
NaF3H4. THF, CH30H
(stereoselective reduction)
2.
H202. ethyl acetate H3Ck~~3
(hydrolysis of the cyclic boronate)
VI
1, diethylmethoxybarane
-
'%O
F
0RH3
CH3
tert-butyl (?)-erythro-(E)-7-
[3-(4-fluoraphenyl)-1 -isopropyl-
indal-2-yl]-3,s-dihydroxy-
hept-6-enoote
(VIl)
NaOH, THF, 10
OC
VII
_____*
@
synthesis of 3-(4-fluorophenyl)-1 -isopropylindole -2-carboxaldehyde
(1):
a-chloro-p- N-isopropyl-
fluoraoceto- aniline
phenone
DMF, 100aC
+
Referencefs):
a
WO
8 402 131 (Sandoz; appl. 18.1 1.1983; USA-prior. 22.1 1.1982, 4.1 l.l983,4.3.1985).
aa
Walkup,
R.E.
et al.: Tetrahedron
Lett.
(TELEAY)
26
(la), 2155-2158 (1985).
b
EP
363 934 (Sandoz; appl. 11.10.1989; USA-prior. 13.10.1983, 22.5.1989).
composition with improved storage stability:
US
5
356 896 (Sandoz; 18.10.1994; appl. 22.12.1992; USA-prior. 12.12.1991).
oral pharmaceutical composition:
EP 547 000 (Sandoz; appl. 8.12.1992; USA-prior. 12.12.1991).
combination with sqr~alene synthase inhibitors:
EP
482
498 (Squibb; appl. 16.10.1991; USA-prior. 19.10.1990).
EP401 705 (Squibb; appl. 1.6.1990; USA-prior. 5.6.1989).
924
F
Fluvoxamine
combination with
ACE
inhibitors:
EP 461 548 (Squibb; appl. 6.6.1991; USA-prior. 11.6.1990).
EP 457 514 (Squibb; appl. 10.5.1991; USA-prior. 15.5.1990).
combination with
niacin
or
probucol:
EP 373 507 (Squibb; appl. 7.12.1982; USA-prior. 12.12.1988).
composition containing
coenzyme Q10:
US 4 933 165 (Merck
&
Co.; 12.6.1990; appl. 18.1.1989; USA-prior. 18.1.1989).
US 4 929 437 (Merck
&
Co.; 29.5.1990; appl. 2.2.1989; USA-prior. 2.2.1989).
Formulation(s):
cps. 21 .O6 rng, 42.12 mg
Trade Name(s):
D: Cranoc (AstraPromed) F: Fractal (Sinbio) GB: Lescol (Novartis)
Loco1 (Novartis Pharma) Lescol (Novartis) USA: Lescol (Novartis)
Fluvoxamine
ATC: N06AB08
Use: antidepressant
RN: 54739- 18-3 MF:
C,,HzIF3N,0z MW: 3 18.34
CN:
(0-5-methoxy- 1
-[4-(trifluorornethyl)phenyl]-
1-pentanone 0-(2-arninoethy1)oxime
hydrogen
maleate
RN: 6171 8-82-9 MF: C,,Hz,F3N202
.
C4H404 MW: 434.41
4'-trifluorornethyl-5- 0-(2-amifloethyl)-
rnethoxyvalerophenone
(1)
hydroxylarnine
H~N-O'~
H3C'~~Cr,
Fluvoxarnine
H~N-''
1
+
HO-NH2
p
2-chloro-
hydroxyl- ethylarnine
arnine
Reference(s):
DE 2 610 886 (Philips Gloeilampenfabrieken; appl. 7.10.1976; prior. 16.3.1976).
US 4 085 225 (Philips Corp.; 18.4.1978; appl. 13.3.1976; NL-prior. 20.3.1975).
NL 7 503 310 (Philips Gloeilampenfabrieken; appl. 20.3.1975).
Formulation(s):
f. c. tabl. 50 rng, 100 mg; tabl. 25 rng, 50 mg, 100 mg (as hydrogen rnaleate)
Trade Name(s):
D: Fevarin (Solvay
GB:
Faverin (Solvay; 1987) USA: Luvox (Solvay)
Arzneirnittel; 1984)
I:
Durnirox (Upjohn)
F: Floxyfral (Solvay Pharma; Fevarin (UCM)
1986) Maveral (Fannades)
6
Folescutol
F
925
Folescutol
ATC: C05C
Use: capillary therapeutic, capillary
protectant
RN:
15687-22-6
MF:
C14HI,N0, MW:
277.28
EINECS:
239-783-0
CN:
6,7-dihydroxy-4-(4-morpholinylmethyl)-2H-l-benzopyran-2-one
hydrochloride
RN:
36002- 19-4
MF: C14H,,NOs
.
HCI MW:
3 13.74
EINECS:
252-83 1-5
HJCYO
Ox~Of~3
+
H2S04
b
::y
mzO
bFi
B
morphollne
H,C
0
1,2,4-tnacetoxybenzene
ethyl
4-chloro-
4
chloramethyl-
Folescutol
acetoacetate
6.7-d~hydroxy-
chrornen-2-one
Reference(s):
FR-M
2
035
(Lab. Dausse; appl.
29.6.1962).
Formulation(s):
drg.
20
mg in comb.
Trade
Name(s):
D:
Detensitral (Kar1spharma)- F: Covalan (Dausse); wfm Tensitral (Dausse)-comb.;
comb.; wfm
wfm
Folic acid
(Pteroylglutamic acid)
ATC:
B03BBOl
Use: antianemic, growth factor
RN: 59-30-3
MF:
C,,lH,,N706
MW:
441.40
EINECS:
200-419-0
LD,&
282
mglkg
(M,
i.
v.);
10
glkg (M, p.0.)
CN:
N-[4-[[(2-amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]-~-glutamic
acid
monosodium
salt
RN:
6484-89-5
MF:
C,,H,,N,NaO,
MW:
463.39
EINECS:
229-348-3
LD,,:
631
mglkg
(M,
i.v.);
526 mglkg
(R,
i.v.)
u
OH
guonidine
ethyl
2,4-diarnino-6-
cyonoacetote
hydroxypyrirnidine
926
F
Folic
acid
H2NQk,,
COOH
NaHS03,
pH
4-5
OH
0
LOOH
6-hydroxy-2.4.5-
1.1,3-tri- N-(4-aminobenzoyl)-
triaminopyrimidine
(I)
chlaroacetone
L-glutamic
acid
(11)
Folic
acid
CHO
NaHS03,
pH
4-5
I
+
Brk~r
+
11
r
2,3-dibromo-
propianaldehyde
NaHSO,,
pH
4-5
I
+
yo
+
11
0
CH,
pyruvaldehyde
Reference(s):
Wailer, C.W. et al.: J. Am. Chem. Soc. (JACSAT)
70, 19 (1948).
Hultquist, M.E. et al.: J. Am. Chem. Soc. (JACSAT)
70, 23 (1948).
Angier, R.B. et al.: J. Am. Chem. Soc. (JACSAT)
70, 25 (1948).
Boothe, J.H. et al.: J. Am. Chem. Soc. (JACSAT)
70, 27 (1948).
US 2 436 073 (American Cyanamid; 1948; appl. 1945).
US 2 442 836 (American Cyanamid; 1948; appl. 1945).
US 2 443 078 (American Cyanamid; 1948; appl. 1945).
US 2 443 165 (American Cyanamid; 1948; appl. 1946).
US 2 444 002 (American Cyanamid;
1948; appl. 1946).
US 2 472 482 (American Cyanamid; -1949; appl. 1947).
US 2 477 426 (American Cyanamid; 1949; appl. 1948).
US 2 547 501 (American Cyanamid; 1951; appl. 1946).
US 2 599 526 (American Cyanamid; 1952; appl. 195 1).
US
2 719 157 (Shionogi; 1955; appl. 1951).
US 2 956 057 (Kongo Kagaku Kabushiki Kaisha; 1960; J-prior. 1955).
use oj'propargyl aldehyde:
US 2 766 240 (Aries Labs.; 1956; appl. 1953).
condensation with a-bromoacroleine:
US 2 476 360 (Parke Davis; 1949; appl. 1946).
alternative synthesis (via
2-amino-6-formyl-4-hydroxy-pteridine):
US 2 786 056 (Merck
&
Co.; 1957; appl. 1954).
US 2 816 109 (Merck
&
Co.; 1957; appl. 1954).
US 2 821 527 (Merck
&
Co.; 1958; appl. 1954).
US 2 821 528 (Merck
&
Co.; 1958; appl. 1954).
US 3 067 200 (Merck
&
Co.; 4.12.1962; prior. 3.5.1954,20.2.1957).
Bieri, J.H.; Viscontini, M.: Helv. Chim. Acta (HCACAV)
56,
2905 (1973).
Fulinic acid
F
927
synthesis via
2-amino-4-hydroxy-6-halogenoin~thylpteridine:
US
2 547 519 (American Cyanamid; 1951; appl. 1946).
US
2 547 520 (Amcrican Cyanamid;
195
1
;
appl. 1946).
US 2 584 538 (American Cyanamid; 1952; appl. 1948).
improved method for synthesis
of
2-amino-4-hydroxy-6-methylptcridine:
GB
1 503 476 (Lonza; appl. 6.1 .lY77; CH-prior. 13.1 .I 976).
US
4
094 874 (Lonza; 13.6.1978; CH-prior. 13.1.1976).
Formulation(s):
amp. 5 mg15 ml; tabl. 5
mg
Trade Name(s):
D:
Folarell (Sanorell)
Fol-ASmedic (Dyckerhoff)
Folsan (Solvay
Arzneimittel)
GB
:
Folsaure Injektionslosung
(Hevert)
Folverlan (Verla)
Lafol (Brenner-Efcka;
LAW)
numerous combination
preparations
F:
Alvityl (Solvay Pharma)-
comb.
Azedavit (Whitehall)-
comb.
Azinc complexe
(Arkopharma)-comb. I:
Carencyl (Riom)-comb.
~16vit Vitamine B9
(Nicholas)-comb.
Forvital (Whitehall)-comb.
Lofenalac (Bristol-Myers
Squibb)-comb.
Plenyl (0berlin)-comb.
Speciafoldine (Specia)
Vivamyne (Whitehall).
comb.
Ferfolic
SV
(Sinc1air)-
comb.
Ferrograd Folic (4bbott)-
comb.
Folex-350 (Shire)-comb.
Galfer
F.A. (Galen)-comb.
Lexpec (Rosemont)
.
Meterfolic (Sinclair)-comb.
Pregaday (Evans)-comb.
Slow-Fe folic (Novartis)-
comb.
numerous combination
preparations
Combetasi
(ISI; as calcium
salt)-comb.
Efargen (Teofarma)-comb.
Epargriseovit (Farrnita1ia)-
comb.
Eparmefolin (Bracco; as
calcium salt)-comb.
Ferrofolin (Farmades; as
calcium salt)-comb.
.
Ferrograd Folic (Abbott)-
comb.
Ferrotre
(Mediolanum)
comb.
Folina (Astra-Simes)
Folinemic (Firma; as
calcium salt)-comb.
Lederfolin (Cyanamid; as
calcium salt)
Oro B 12 (Ripari-Gero)-
comb.
Tonofolin
(Zyma; as
calcium salt)
J:
Foliamin (Takeda)
Folical (Shionogi)
USA: Bevitamcl (Westlake)
Cefol (Abbott)
Folic (Lederle)
Materna (Lederle)
various generic
preparations
Folinic
acid
(Citrovorum factor)
ATC: A04A; V03AB
Use: antianemic, growth factor, antidote
(as calcium salt, at overdose of folic
acid antagonists)
RN: 58-05-9 MF: C20H23N707
MW.
473.45 EINECS: 200-361-6
CN:
N-[4-[[(2-amino-5-formyl-l,4,5,6,7,8-hexahydra-4-oxo-6-pteridinyl)methyl]amino]benzoyl]-~-glutamic
acid
calcium
salt (1:l) (leucovorin calcium)
RN: 1492-1 8-8 MF: C,,,H,,CaN,O,
MW.
5 1 1.5
1
EINECS: 21 6-082-8
LD,,:
732 mglkg
(M,
i.v.); >7 glkg (M, pa.);
>8
gkg (R, PA.)
dcium
salt (1:l) pentahydrate
RN: 6035-45-6 MF: C,,H,ICaN,07
.
SH,O MW: 601 .58
928
F
Fomepizole
HN N
N
0
0
ZJNUQ
H
+
HCOOH
H3c~oJLH3
40
OC
OH
/
N,,,cCOOH
I
folk
ocid
(q.
v.)
Reference(s):
US 2 741 608 (Research Corp.; 1956; prior. 1950).
DOS 2 836 599 (US Department of Commerce; appl. 22.8.1978; USA-prior. 22.8.1977).
Temple, C. et al.:
J.
Med. Chem. (JMCMAR) 22,731 (1979).
10-farrnylfolic
acid
(I)
Formulution(s):
amp.
3
mg, 5 mg, 6 mg, 10 mg, 15 mg, 30 mg, 50 mg; cps. 5 mg; tabl. 15 mg, 25 mg (as
calcium salt); amp. 1.5 mg, 3 mg, 15 mg, 30 mg, 350 mg; cps. 15 mg; powder 50 mg,
100mg,
200 mg, 300 mg; tabl. 15 mg; vial 100 mg, 200 mg, 300 mg (as calcium salt pentahydrate)
Folinic
acid
Trade Nume(s):
D:
Calciurnfolinat (Rh6ne-
Poulenc); wfm
Leucovorin (Lederle); wfm
Rescuvolin (medac);
wfrn
F:
Elvorine (Wyeth-Lederle)
Folinate de calcium
(Aguettant)
Folinoral (Therabel Lucien
Pharma)
Lederfoline
(Wyeth-
Lederle)
Osfolate (ASTA Medica)
Perfolate (ASTA Medica)
GB:
Calcium Leucovorin
(Lederle); wlm
Refolinon (Pharrnacia
&
Upjohn)
Rescufolin (Nordic); wfin
I:
Adinepar (Leben1s)-comb.
Hepafactor (S~gma-Tau)-
comb.
Rekord
B
12 complex
(Sigma-Tau)-comb.
J:
Leucovorin (Lederle)
USA: Calcium Leucovorin
(Lederle); wfm
Fomepizole
Use: antidote for ethlene glycol,
competitive inhibitor of alcohol
dehydrogenase
RN: 7554-65-6 MF: C4H6N2
MW:
82.11 EINECS: 231-445-0
CN: 4-Methyl-1H-pyrazole
Pd-Mg0.
AI2O3,
25'C
or
N2H4
-H20
I,,
Nal,
H20.
H2S04
hydrate
CH3
2-methyl-2- 4.5-dihydro- Fomepizole
propenal
4-methyl-
pyrozole
Fominoben
F
929
Reference(s):
Pechrnann, H.; Burkard,
E.:
Ber. Dtsch. Chem. Ges. (BDCGAS)
33,
3590 (1900).
Hoyce, D.S. eta].:
J.
Org. Chem. (JOCEAH)
20,
1681 (1955).
Momose, T. et a].: Heterocycles (HTCYAM)
30,
789 (1990).
DE4 328 228 (BASF; prior. 23.8.1993).
US
5 569 769 (BASF; 29.10.1996; D-prior. 23.8.1993).
DE
3 918 979 (BASF; prior. 10.6.1989).
EP366 328 (Nissan Chem. Ind.; appl. 17.10.1989; J-prior. 26.10.1988).
Formulation(s):
vials, 1 glml;
1.5
ml
Tmde Name(s):
USA:
Antizol (Orphan Medical;
1998)
Fominoben
ATC: ~06
Use: antitussive, respiratory analeptic
RN:
18053-31-1
MF:
C2,H24ClN30, MW: 401.89 EINECS: 241-964-4
CN:
N-[3-chloro-2-[[methy1[2-(4-morpholinyl)-2-oxoethyl]amino]methyl]phenyl]benzamide
monohydrochloride
RN:
24600-36-0 MF: C2,H24CIN,03
.
HCI MW: 438.36
EINECS:
246-344-7
benzoyl
3-chloro-
chloride 2-methylaniline
0
0-Br
0
N-bromo-
succinimide
Reference(s):
DE 1 795 259 (Thomae; appl. 13.7.1966).
sorcosine
Kriiger,
G.
et al.: Arzneim Forsch. (ARZNAD) 23,290 (1973)
Fominoben
Formulation(s):
amp. 40 mgl5 ml; drg 160 mg (as hydrochloride)
morpholide
Trade Namels):
D: Broncho-Noleptan Noleptan (Thomae); wfm Tussirama (Serpero); wfm
(Thomae); wfm I: Terion (Lusafarmaco); wfm
930
F
Fomocaine
Fomocaine
ATC: R02AD
Use: local anesthetic
RN: 17692-39-6 MF: C20H25N02 MW: 3 11.43
CN:
4-[3-[4-(phenoxymethyl)phenyl]propyl]morpholine
3-phenyl-1-
proponol
3-phenylpropyl
chloride
0';""
0-
3-(4-phenoxymethyl- line
(n)
phenyl)propyl chloride
Fomocoine
H,C,MqBr
bromide
4-bromomethyl- 4-phenoxymethyl-
benzonitrile benzonitrile
Roney-Ni
4'-phenoxymethyl-
propiophenone
(In)
Reference(s):
a
Oelschlager,
H.:
Arzneirn Forsch. (ARZNAD)
9,
313 (1959).
GB 786 128 (Prornonta; appl. 15.1 1.1955; D-prior. 15.1 1.1954).
b
Oelschlager,
H.
et al.: Arzneim Forsch. (ARZNAD)
27,
1625 (1 977).
pharmacology:
Nieschulz, 0. et al.: Arzneim Forsch. (ARZNAD) 8,539 (1958).
Formularion(s):
cream 4 gI100 g (4
%);
ointment 4 g1100 g (4
%)
Trade Name(s):
D: Brand- und Wund-Gel Erbocain (Heilit); wfrn
Herit (Engelhard)-comb.; Erboproct (Hei1it)-comb.;
wfrn wfrn